How to use
* Images used are not actual patients
DOSAGE AND ADMINISTRATION
1. Radiation Safety – Drug Handling
After radiolabeling, handle Technetium Tc 99m Succimer Injection with appropriate safety measures to minimize radiation exposure. Use waterproof gloves, effective radiation shielding, and other appropriate safety measures when preparing and handling Technetium Tc 99m Succimer Injection.
Radiopharmaceuticals should be used by or under the control of physicians who are qualified by specific training and experience in the safe use and handling of radionuclides, and whose experience and training have been approved by the appropriate governmental agency authorized to license the use of radionuclides.
2. Patient Preparation
Instruct patients to drink a sufficient amount of water to ensure adequate hydration prior to administration of Technetium Tc 99m Succimer Injection and to continue to drink and void frequently following administration to reduce radiation exposure.
3. Recommended Dosage
Adults
The recommended amount of radioactivity of Technetium Tc 99m Succimer Injection for renal parenchymal imaging in adults is 74 MBq to 222 MBq (2 mCi to 6 mCi) by intravenous injection (bolus).
Pediatric Patients
The recommended amount of radioactivity of Technetium Tc 99m Succimer Injection for renal parenchymal imaging in pediatric patients is 1.85 MBq/kg (0.05 mCi/kg) of body weight with a range of 19 MBq to 74 MBq (0.5 mCi to 2 mCi) by intravenous injection (bolus). Weight based pediatric dosing is shown in Table 1.
Table 1 Recommended Radioactivity of Technetium Tc 99m Succimer Injection for Pediatric Patients by Body Weight
| Body Weight (kg) | Recommended Radioactivity MBq (mCi) | Body Weight (kg) | Recommended Radioactivity MBq (mCi) |
| less than 11 kg | 19 MBq (0.5 mCi) | 25 to 26 | 49 MBq (1.3 mCi) |
| 11 to 12 | 21 MBq (0.6 mCi) | 27 to 28 | 52 MBq (1.4 mCi) |
| 13 to 14 | 26 MBq (0.7 mCi) | 29 to 30 | 56 MBq (1.5 mCi) |
| 15 to 16 | 30 MBq (0.8 mCi) | 31 to 32 | 59 MBq (1.6 mCi) |
| 17 to 18 | 33 MBq (0.9 mCi) | 33 to 34 | 63 MBq (1.7 mCi) |
| 19 to 20 | 37 MBq (1 mCi) | 35 to 36 | 67 MBq (1.8 mCi) |
| 21 to 22 | 41 MBq (1.1 mCi) | 37 to 38 | 70 MBq (1.9 mCi) |
| 23 to 24 | 44 MBq (1.2 mCi) | 39 or greater | 74 MBq (2 mCi) |
4. Drug Preparation
Prepare Technetium Tc 99m Succimer Injection according to the following procedure using asceptic technique:
- Wear waterproof gloves.
- Place the kit vial in lead shielding and disinfect the stopper (allow disinfectant to dry).
- Use a sterile syringe to transfer 5 mL sodium pertechnetate Tc 99m injection obtained from a technetium Tc 99m generator with a maximum activity of 1,480 MBq (40 mCi) to the vial. The volume of sodium pertechnetate Tc 99m injection may be adjusted to 5 mL prior to adding to the kit vial using 0.9% sodium chloride injection, USP.
- Use the same syringe to withdraw the appropriate gas volume from the vial for pressure compensation.
- Lightly shake the vial to completely dissolve the powder. Ensure the stopper is well moistened.
- Incubate the vial for 10 minutes at controlled room temperature 20°C to 25°C (68°F to 77°F).
- Measure the product activity in a dose calibrator; complete the radiolabeled product vial label and affix to the vial shield.
- Check radiochemical purity [see Package Insert section 2.5]
- Use Technetium Tc 99m Succimer Injection within 4 hours and store at controlled room temperature 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F).
5. Radiochemical Purity Determination
Determine the radiochemical purity of Technetium Tc 99m Succimer Injection as follows:
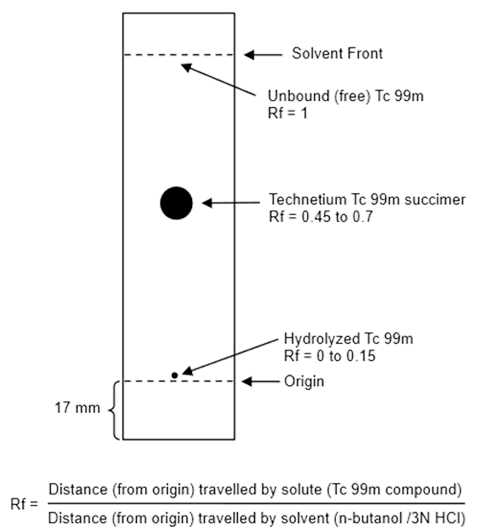
- Activate a 65 x 95-mm silicic acid thin-layer chromatographic (TLC) plate by heating at 100°C to 110°C (212°F to 230°F) for 30 minutes.
- Cool the TLC plate over silica gel and immediately apply 1 microL of Technetium Tc 99m Succimer Injection about 17 mm from one end of the TLC plate and allow to dry. If necessary, the Technetium Tc 99m Succimer Injection may be diluted with 0.9% sodium chloride injection USP, to a radioactive concentration of 18.5 MBq to 370 MBq (0.5 mCi to 10 mCi) per mL.
- Develop the TLC plate over a period of about 30 minutes to 45 minutes by ascending chromatography, using a solution of n-butanol saturated with 0.3 N hydrochloric acid (see Note 1). Air-dry the developed TLC Plate.
- Determine the radioactive distribution on the TLC plate by scanning the chromatogram with a radiochromatographic scanner having a suitably collimated radiation detector.
- The radioactivity associated with technetium Tc 99m succimer is at Rf between 0.45 and 0.7, hydrolyzed Tc 99m is located at the origin (Rf 0 to 0.15) and the unbound Tc 99m is located at the solvent front (Rf 1).
f. Calculate radiochemical purity using the following formula:

g. Technetium Tc 99m Succimer Injection preparation with not less than 85% radiochemical purity is suitable for administration. Discard preparation with less than 85% radiochemical purity.
Note 1: To prepare the n-butanol saturated with 0.3 N hydrochloric acid solution, place 50 mL of 0.3 N HCl and 50 mL of n-butanol in an Erlenmeyer flask. Place the mixture in an ultrasonic bath for 2 hours, during which time the solution heats up to about 50°C. Cool the solution to room temperature. After about 30 minutes to 45 minutes the phase separation of the mixture will complete. Collect the upper phase of the mixture and discard the lower phase. The solution is stable for up to 7 days when stored at controlled room temperature 20°C to 25°C (68°F to 77°F).
6. Administration
Prior to use, visually inspect the prepared Technetium Tc 99m Succimer Injection behind a lead glass shield. Only use solutions that are clear without visible particles.
In making dosage calculations, correction is to be made for radioactive decay. The radioactive half-life of Tc 99m is 6.0 hours.
Using a sterile shielded syringe, aseptically withdraw the prepared Technetium Tc 99m Succimer Injection, and measure the radioactivity in the syringe using a dose calibrator, prior to administration. Ensure that the injected radioactivity is within ±10% of the recommended dose.
Discard unused portion. Handle and dispose radioactive material in accordance with applicable regulations.
7. Image Acquisition
The patient should be placed in the prone or supine position, as required by scanning equipment characteristics. Begin image acquisition 1 hour to 4 hours after the intravenous administration of Technetium Tc 99m Succimer Injection.
Delay image acquisition up to 6 hours to 24 hours in patients with severely reduced glomerular filtration rate (eGFR). A specific eGFR threshold at which to delay imaging has not been established.
8. Radiation Dosimetry
The estimated absorbed radiation doses to an average adult and pediatric patients are shown in Table 2.
Table 2 Estimated Radiation Absorbed Dose per Unit of Administered Radioactivity in Selected Organs and Tissues after a Technetium Tc 99m Succimer Injection Dose
| Absorbed Dose per Unit of Activity Administered (mGy/MBq) | |||||
| Organ | Adults | 15 years | 10 years | 5 years | 1 year |
| Adrenals | 0.012 | 0.016 | 0.024 | 0.035 | 0.06 |
| Bladder wall | 0.018 | 0.023 | 0.029 | 0.031 | 0.057 |
| Bone surface | 0.005 | 0.0062 | 0.0092 | 0.014 | 0.026 |
| Brain | 0.0012 | 0.0015 | 0.0025 | 0.004 | 0.0072 |
| Breasts | 0.0013 | 0.0018 | 0.0028 | 0.0045 | 0.0084 |
| Gall bladder | 0.0083 | 0.01 | 0.014 | 0.022 | 0.031 |
| Stomach wall | 0.0052 | 0.0063 | 0.01 | 0.014 | 0.02 |
| Colon | 0.005 | 0.0063 | 0.01 | 0.014 | 0.024 |
| Intestine | 0.0043 | 0.0055 | 0.0082 | 0.012 | 0.02 |
| Upper large intestine | 0.005 | 0.0064 | 0.095 | 0.014 | 0.023 |
| Lower large intestine | 0.0035 | 0.0043 | 0.0065 | 0.0096 | 0.016 |
| Heart | 0.003 | 0.0038 | 0.0058 | 0.0086 | 0.014 |
| Kidneys | 0.18 | 0.22 | 0.3 | 0.43 | 0.76 |
| Liver | 0.0095 | 0.012 | 0.018 | 0.025 | 0.041 |
| Lungs | 0.0025 | 0.0035 | 0.0052 | 0.008 | 0.015 |
| Muscles | 0.0029 | 0.0036 | 0.0052 | 0.0077 | 0.014 |
| Oesophagus | 0.0017 | 0.0023 | 0.0034 | 0.0054 | 0.0094 |
| Ovaries | 0.0035 | 0.0047 | 0.007 | 0.011 | 0.019 |
| Pancreas | 0.009 | 0.011 | 0.016 | 0.023 | 0.037 |
| Red marrow | 0.0039 | 0.0047 | 0.0068 | 0.009 | 0.014 |
| Skin | 0.0015 | 0.0018 | 0.0029 | 0.0045 | 0.0085 |
| Spleen | 0.013 | 0.017 | 0.026 | 0.038 | 0.061 |
| Testes | 0.0018 | 0.0024 | 0.0037 | 0.0053 | 0.01 |
| Thymus | 0.0017 | 0.0023 | 0.0034 | 0.0054 | 0.0094 |
| Thyroid | 0.0015 | 0.0019 | 0.0031 | 0.0052 | 0.0094 |
| Uterus | 0.0045 | 0.0056 | 0.0083 | 0.011 | 0.019 |
| Remaining organ | 0.0029 | 0.0037 | 0.0052 | 0.0077 | 0.014 |
| Effective Dose per unit of activity administered (mSv/MBq) | 0.0088 | 0.011 | 0.015 | 0.021 | 0.037 |
For important risk and use information, please see Full Prescribing information.
